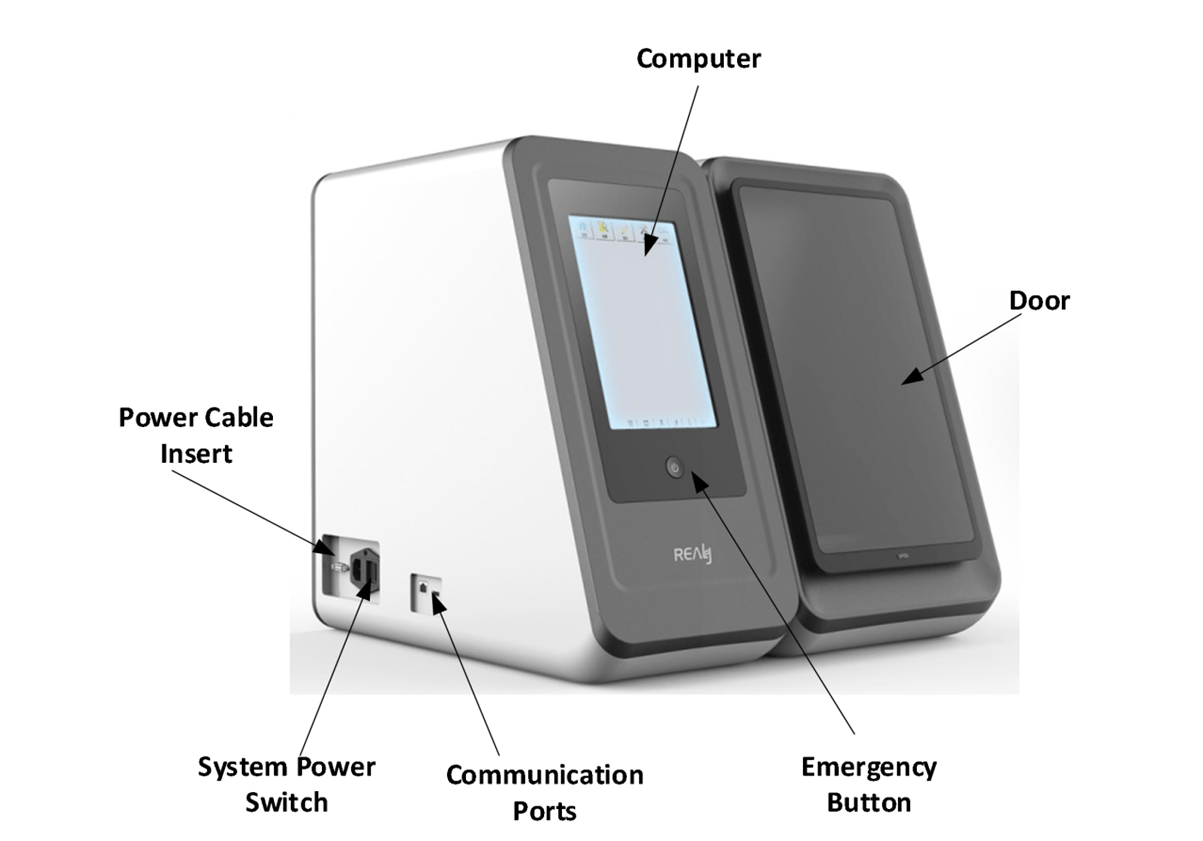
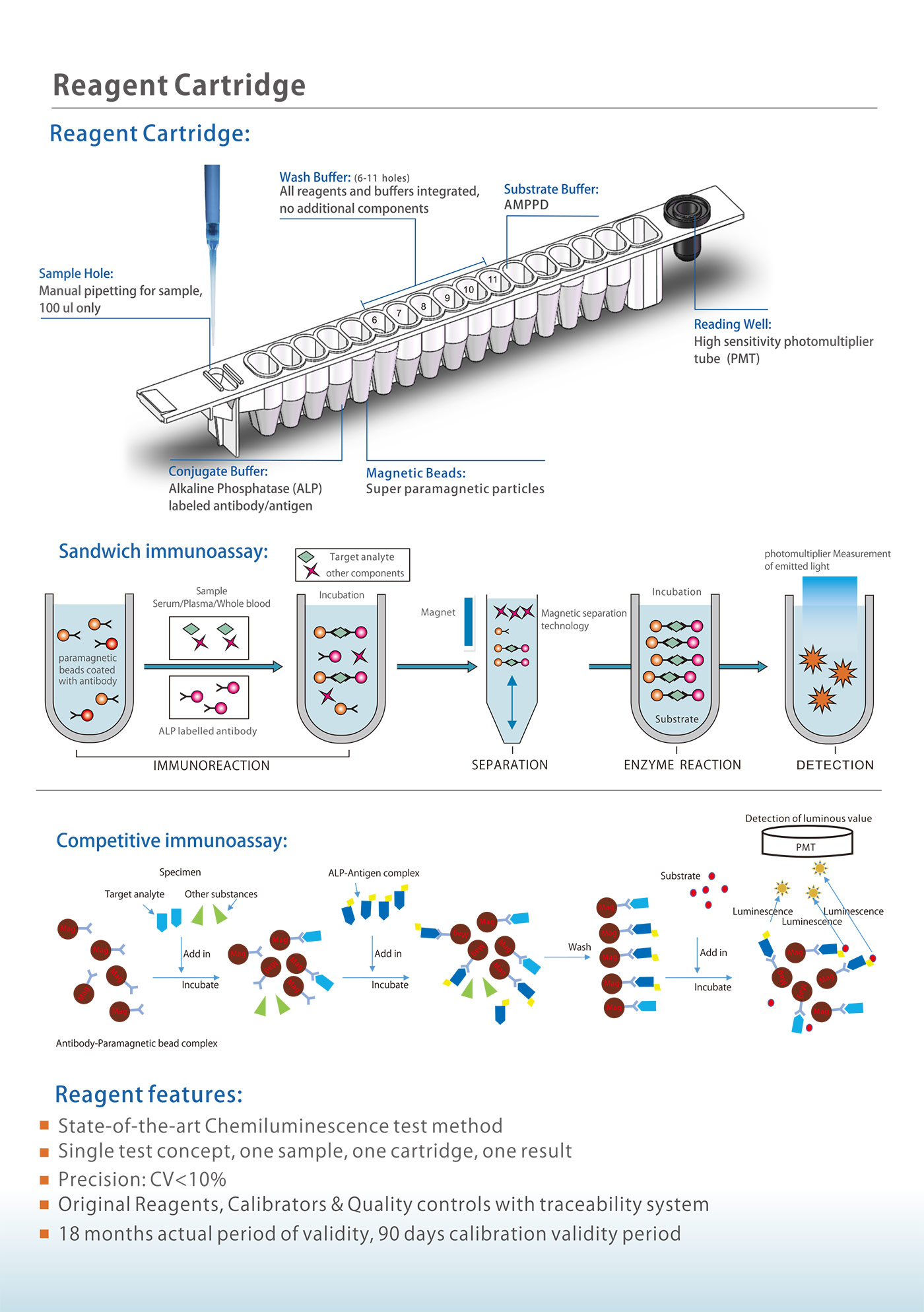
The analyzer uses enzymatic chemiluminescence immunoassay and is used together with the supporting detection reagents to clinically be used for qualitative or quantitative detection of analytes in serum, plasma, whole blood or urine samples derived from human body, including hormones, tumor-associated antigens, infectious diseases, autoimmunity, allergens, allergen related items. The analyzer uses PMT to detect the photonics, convert the optical signal to digital signal after
amplification and discrimination. Finally the measured value is substituted into the master curve of the reagent to calculate the concentration.